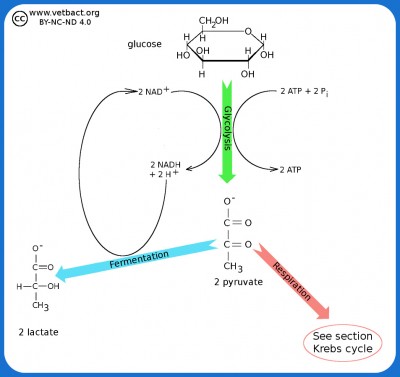
Glycolysis and Fermentation
The figure shows a very simplified picture of bacterial glycolysis and fermentation where the final product is lactate (the ionized form of lactic acid). The net gain of glycolysis is 2 ATP and 2 NADH per glucose molecule. However, the two molecules of NADH are used in the fermentation of pyruvate to lactate. Pyruvate acts as an electron acceptor and can oxidize NADH to NAD+, which is then reused to re-oxidize glucose.
Image: Karl-Erik Johansson (BVF, SLU) - Click on the image to enlarge it.
Introduction
All bacteria of importance in veterinary and human medicine are chemoorganoheterotrophic, which means that they get their energy from organic carbon compounds, which are also used as carbon source. The only exception is cyanobacteria, which are photolithoautotrophic, i.e. they get their energy from light and they use inorganic carbon in the form of CO2 (carbon dioxide) as carbon source.
Bacteria can thus extract energy through oxidation of carbohydrates (especially glucose) and these bacteria are said to be saccharolytic. Some other bacteria can instead use amino acids or lipids as an energy source, but this is less common and these bacteria are said to be asaccharolytic. Carbohydrates are also included in a variety of cellular processes in bacteria and bacterial carbohydrate metabolism is highly diversified. This is the reason why bacteria can grow and thrive in practically every environment on our planet.
Glycolysis
Glucose, which is a 6-carbon monosaccharide (hexose monosaccharide), is one of the most important carbohydrate molecules that bacteria can extract energy from. This is done by an oxidation process, called glycolysis, and in the glycolysis, glucose is broken down into pyruvate. Bacteria can also break down e.g. lactose and mannitol to pyruvate by glycolysis. Lactose is a disaccharide, which consists of two hexose monosaccharides (galactose and glucose). Mannitol is a hexose monosaccharide alcohol. If a bacterium has the right enzyme systems, then these carbohydrates can also be oxidized by glycolysis.
Under anaerobic conditions, pyruvate, can be converted to a variety of products, depending on the enzyme system of the microorganism in question. This process is called fermentation and by examining which carbohydrates a particular bacterium can ferment and which end products are formed, one can identify the bacterium in question.
Some bacteria can also convert the amino acid tryptophan to pyruvate if they have the enzyme typtophanase (can be detected by the indole test). Puruvat can then take various pathways in the metabolism, depending on whether O2 (oxygen) is available and depending on which enzyme system the bacterium has. If O2 is missing and if the bacterium is facultatively anaerobic, acid tolerant or anaerobic, puruvate can be further converted by fermentation.
Fermentation
Fermentation is a metabolic process that does not require O2 and can be utilized by many different bacteria. The fermentation in combination with the glycolysis results in the production of ATP, which is the most important form of energy for bacteria and other organisms. Examples of end products after the fermentation are: lactic acid (lactobacilli), ethanol (yeast), 2,3-butanediol (Klebsiella spp.), formic acid (Shigella spp.), butyric acid (Clostridium sp.) and mixed acids (E. coli m. al.). Glycolysis in combination with fermentation is not an efficient process for recovering energy because the carbohydrate is not completely oxidized to CO2 and H2O. Therefore, only 2 ATP molecules are formed for each carbohydrate molecule that is oxidized and facultative anaerobes always grow better in the presence of O2.
In the sections on the Krebs Cycle and on the Electron transport chain it is described how the chemical energy in pyruvate can be more efficiently extracted.